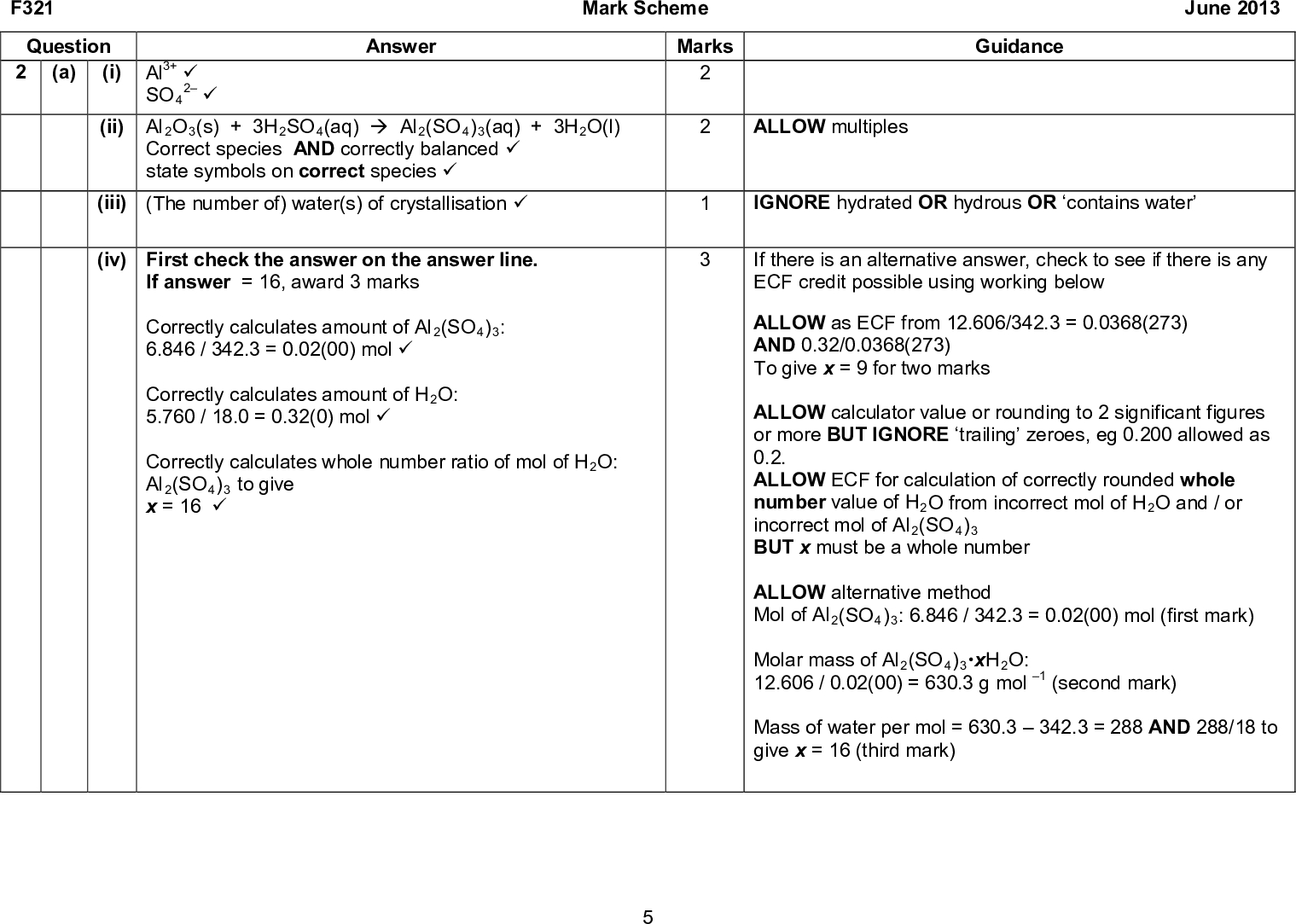
Moles Al2(SO4)3 B) What Is The Molar Mass Of Al2(SO4)3, To The (If you've got moles and molecular weight you can get to grams.) _ g Al2(SO4)3. d) How many moles of aluminum ions are present in the solution?29. The molar mass of Al2(SO4)3is…a. 33. What can be said about 1.00 mol NaCl and 1.00 mol NaNO3? a. They are equal in mass. b. They contain the same number of particles. c. Their molar masses are equal. d. They have the same atomic mass.Aluminium sulfate is a chemical compound with the formula Al2(SO4)3. It is soluble in water and is mainly used as a coagulating agent (promoting particle collision by neutralizing charge)...Number of moles=MolecularmassMass Given, mass of Al2 (SO4 )3 =60g Molecular mass of Al2 (SO4 )3 =2×27+3(32+4×16) Molecular mass of nitrogen is 28. Its atomic mass is 14. Find the atomicity of nitrogen. View solution. How many grams of Cl2 gas will be obtained by the complete reaction of 31.5...Calculate the molar mass of Al2(SO4)3 in grams per mole or search for a chemical formula or substance. This is how to calculate molar mass (average molecular weight), which is based on isotropically weighted averages. This is not the same as molecular mass, which is the mass of a...
The molar mass of Al 2 SO 4 3 is a 7505 gmol c 15003... | Course Hero
What is the molar mass of Al2(SO4)3? 342.17 g/mol. How are a mole and a dozen similar? Each names a given number of objects. Which statement best describes a mole? It contains 6.02 x 10^23 particles of a given substance. The mole concept will most likely be used in.Therefore the molar mass of NaOH (Sodium Hydroxide) is 40.00g. The molar mass of any element is the atomic mass.Formula: Al2(SO4)3 Enter a chemical formula to calculate molar mass,The molar mass calculator can be used in Chemical industry and medicine industry. H2SO4= H2SO4.Molar mass and elemental composition calculator. Relative atomic masses are expressed with five significant figures. The relative atomic mass indicates how many times larger the mass of a given atom is than 1/12 the mass of the 12C carbon J. Meija et al., Atomic Weights of the Elements 2013.
Aluminium sulfate - Wikipedia
Molar mass calculator computes molar mass, molecular weight and elemental composition of any given compound. Enter a chemical formula to calculate its molar mass and elemental composition: Molar mass of Al2(SO4)3 is 342.1509 g/mol Compound name is aluminium sulfate.To find the molar mass of aluminum, you need to follow the algorithm: Prepare a periodic table to find out the valence of a given element and atomic M = Ar, Ar (Al) = Al · 1 mol = 26,98 g/mol. If your substance, for example, would be aluminum sulfate, then its chemical formula looks like this Al2 SO4)...Explanation of how to find the molar mass of Al2(SO4)3 (Aluminum Sulfate).A few things to consider when finding the molar mass for Al2(SO4)3:- make sure you...Here is ur answer. Molar mass of.mirceavaman mirceavaman. MAl2(SO4)3=2*MAl+3*MS+12*MO=2*27+3*32+12*16=54+96+192=342g/mol.
Molar mass of Al2(SO4)3 = 342.150876 g/mol
This compound is also known as Aluminium Sulfate.
Convert grams Al2(SO4)Three to moles or moles Al2(SO4)3 to grams
Molecular weight calculation:26.981538*2 + (32.065 + 15.9994*4)*3
Element Symbol Atomic Mass # of Atoms Mass %Aluminium Al 26.981538 2 15.772%Oxygen O 15.9994 12 56.113%Sulfur S 32.065 3 28.115%In chemistry, the formula weight is a quantity computed through multiplying the atomic weight (in atomic mass devices) of every part in a chemical formula by the quantity of atoms of that component provide within the components, then including all of those merchandise together.
A common request in this web site is to transform grams to moles. To complete this calculation, you need to know what substance you are attempting to transform. The reason is that the molar mass of the substance impacts the conversion. This web site explains find molar mass.
The atomic weights used on this website come from NIST, the National Institute of Standards and Technology. We use the most typical isotopes. This is how you can calculate molar mass (moderate molecular weight), which is in keeping with isotropically weighted averages. This is not the same as molecular mass, which is the mass of a single molecule of well-defined isotopes. For bulk stoichiometric calculations, we are usually determining molar mass, which will also be known as same old atomic weight or moderate atomic mass.
Using the chemical method of the compound and the periodic table of components, we can upload up the atomic weights and calculate molecular weight of the substance.
Finding molar mass starts with gadgets of grams in line with mole (g/mol). When calculating molecular weight of a chemical compound, it tells us what number of grams are in a single mole of that substance. The formulation weight is solely the weight in atomic mass units of all the atoms in a given formula.
Formula weights are particularly useful in determining the relative weights of reagents and products in a chemical reaction. These relative weights computed from the chemical equation are often referred to as equation weights.
If the system used in calculating molar mass is the molecular system, the formula weight computed is the molecular weight. The proportion through weight of any atom or staff of atoms in a compound will also be computed by dividing the overall weight of the atom (or team of atoms) in the components by means of the components weight and multiplying by 100.
CHEM 1211K Homework Chapter 3 Name: Student ID: Balance the

What is the formula mass of Al2(SO4)3? How is this ...
PPT - Unit # 3 Stoichiometry: Calculations with Chemical ...

how much maximum mass of aluminium can be obtained from ...

Stoichiometry • • • • • • • • Atomic Mass The Mole concept ...

OCR A Jun 2013 Paper 1 Q2 (with explained solutions)
Molar Mass Of All Elements In Periodic Table | www ...

PPT - Example 3.1 Molecular and Empirical Formulas ...
What is the molecular mass of H2SO4? - Quora
How To Get Molar Mass From Molecular Weight - Mang Temon

How To Get Molar Mass From Molecular Weight - Mang Temon

Helium: Helium Gas Molar Mass

PPT - Chemical Formulas and Chemical Compounds PowerPoint ...

ps01_S15 - Problem Session#1 CH101-001 A J Petrovich S15 1 ...

Periodic Table With Atomic Mass Rounded To Hundredths ...
3.11 Molecular Mass of Al2(Cr2O7)3 - YouTube

How To Get Molar Mass From Molecular Weight - Mang Temon

Answered: What is the formula weight of the… | bartleby
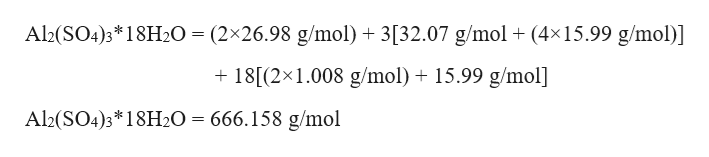
why ice at 0 degree Celsius is more effective in cooling ...

Periodic Table Sulfur Atomic Mass | www.microfinanceindia.org

molarity of K3Fe(CN)6 in solution - HomeworkLib

0 Comment to "What Is The Molecular Mass Of Al2(SO4) 3? - Quora"
Post a Comment